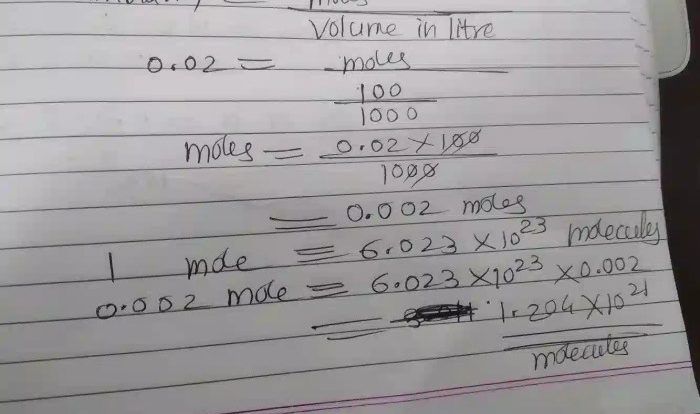Classify these structures by the hybridization of the central atom sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. This comprehensive guide delves into the fascinating realm of hybridization, providing a clear and concise exploration of its impact on molecular geometry, bond properties, and chemical reactivity.
The concept of hybridization, a cornerstone of modern chemistry, unveils the intricate dance of atomic orbitals, shaping the very essence of molecules. By understanding the hybridization of the central atom, we gain profound insights into the behavior and properties of countless chemical compounds, unlocking the secrets that govern their existence and interactions.
Hybridization of Central Atom: Classify These Structures By The Hybridization Of The Central Atom

Hybridization is a theoretical concept in chemistry that describes the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. It helps explain the bonding and geometry of molecules and ions. The hybridization of the central atom refers to the type of hybrid orbitals formed by the central atom in a molecule.The
concept of hybridization was developed by Linus Pauling in the 1930s to explain the geometry of molecules such as methane (CH4) and water (H2O). According to the hybridization theory, the central atom’s atomic orbitals undergo hybridization to form a set of equivalent hybrid orbitals.
These hybrid orbitals then overlap with the orbitals of other atoms to form covalent bonds.
Sp Hybridization
In sp hybridization, one s orbital and one p orbital of the central atom combine to form two sp hybrid orbitals. These hybrid orbitals are linear in shape and are oriented at 180° from each other. Molecules with sp hybridized central atoms have a linear geometry, such as carbon dioxide (CO2) and beryllium dichloride (BeCl2).
Sp2 Hybridization, Classify these structures by the hybridization of the central atom
In sp2 hybridization, one s orbital and two p orbitals of the central atom combine to form three sp2 hybrid orbitals. These hybrid orbitals are trigonal planar in shape and are oriented at 120° from each other. Molecules with sp2 hybridized central atoms have a trigonal planar geometry, such as boron trifluoride (BF3) and formaldehyde (H2CO).
Sp3 Hybridization
In sp3 hybridization, one s orbital and three p orbitals of the central atom combine to form four sp3 hybrid orbitals. These hybrid orbitals are tetrahedral in shape and are oriented at 109.5° from each other. Molecules with sp3 hybridized central atoms have a tetrahedral geometry, such as methane (CH4) and ammonia (NH3).
Classifying Structures by Hybridization
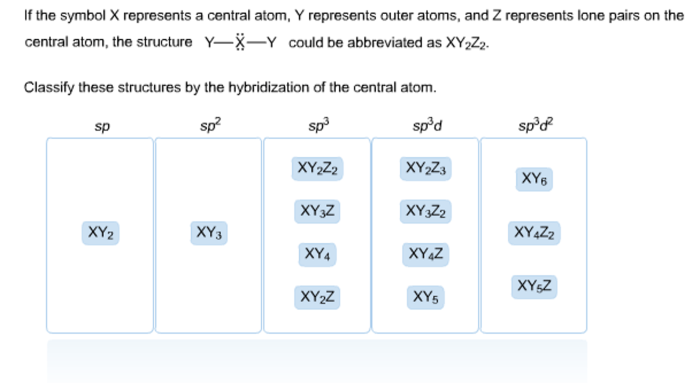
The hybridization of the central atom can be used to classify molecules and ions. Molecules with sp hybridized central atoms have a linear geometry, molecules with sp2 hybridized central atoms have a trigonal planar geometry, and molecules with sp3 hybridized central atoms have a tetrahedral geometry.The
following table provides examples of molecules with sp, sp2, and sp3 hybridized central atoms:| Hybridization | Central Atom | Molecular Geometry | Examples ||—|—|—|—|| sp | C | Linear | CO2, BeCl2 || sp2 | B | Trigonal Planar | BF3, H2CO || sp3 | C | Tetrahedral | CH4, NH3 |
Applications of Hybridization

The concept of hybridization is significant in predicting molecular properties such as bond length, bond strength, and reactivity.
- Bond Length:The hybridization of the central atom influences the bond length. For example, sp hybridized carbon atoms form shorter and stronger bonds than sp2 hybridized carbon atoms, which in turn form shorter and stronger bonds than sp3 hybridized carbon atoms.
- Bond Strength:The hybridization of the central atom also affects the bond strength. Hybrid orbitals with more s-character are more stable and form stronger bonds. For example, sp hybridized bonds are stronger than sp2 hybridized bonds, which are stronger than sp3 hybridized bonds.
- Reactivity:The hybridization of the central atom can influence the reactivity of a molecule. Molecules with sp hybridized central atoms are more reactive than molecules with sp2 hybridized central atoms, which are more reactive than molecules with sp3 hybridized central atoms.
Hybridization in Organic Chemistry
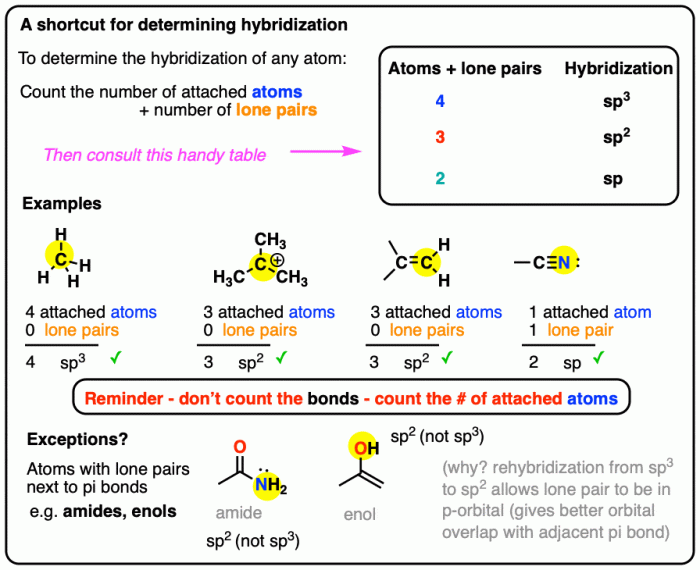
The concept of hybridization is particularly important in organic chemistry. The hybridization of the carbon atom in a functional group determines the reactivity and properties of the molecule. The following table compares the hybridization of central atoms in different functional groups:| Functional Group | Central Atom Hybridization ||—|—|| Alkanes | sp3 || Alkenes | sp2 || Alkynes | sp || Alcohols | sp3 || Aldehydes | sp2 || Ketones | sp2 || Carboxylic Acids | sp2 |The hybridization of the carbon atom in a functional group affects the chemical reactivity of the molecule.
For example, sp2 hybridized carbon atoms in alkenes and alkynes are more reactive than sp3 hybridized carbon atoms in alkanes. This difference in reactivity is due to the different shapes and energies of the hybrid orbitals.
FAQ Section
What is hybridization?
Hybridization is the process of combining atomic orbitals to form new hybrid orbitals with different shapes and energies.
How does hybridization affect molecular geometry?
Hybridization determines the arrangement of atoms around the central atom, influencing the overall molecular geometry.
What are the different types of hybridization?
The most common types of hybridization are sp, sp2, and sp3, which result in linear, trigonal planar, and tetrahedral molecular geometries, respectively.